Agar
Agar or agar-agar was discovered in the late 1650s or early 1660s by Mino Tarozaemon in Japan, where it is called kanten. Agar is derived from the polysaccharide agarose, which forms the supporting structure in the cell walls of certain species of algae, and which is released on boiling.
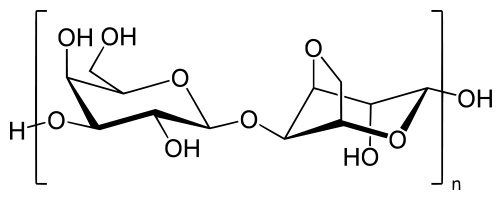
Agarose is a polysaccharide polymer material, generally extracted from seaweed. Agarose is a linear polymer made up of the repeating unit of agarobiose, which is a disaccharide made up of D-galactose and 3,6-anhydro-L-galactopyranose. Agarose is one of the two principal components of agar, and is purified from agar by removing agar's other component, agaropectin.
Below shows a fragment of the agarose chain --
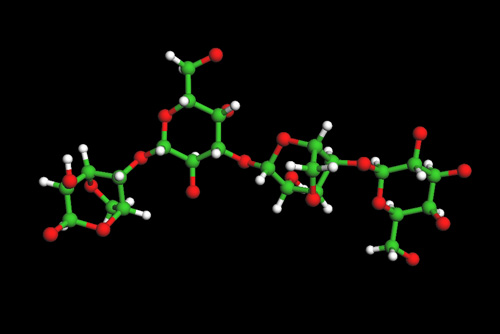
Molecular Structure of Agar Agar
Agarose, the gelling fraction, is a neutral linear molecule essentially free of sulfates, consisting of chains of repeating alternate units of ß-1,3-linked- D-galactose and a-1,4-linked 3,6-anhydro-L-galactose. Agaropectin, the non gelling fraction, is a sulfated polysaccharide (3% to 10% sulfate), composed of agarose and varying percentages of ester sulfate, D-glucuronic acid, and small amounts of pyruvic acid. The proportion of these two polymers varies according to the species of seaweed. Agarose normally represents at least two-thirds of the natural agar-agar.
Agarose, the gelling fraction, is a neutral linear molecule essentially free of sulfates, consisting of chains of repeating alternate units of ß-1,3-linked- D-galactose and a-1,4-linked 3,6-anhydro-L-galactose. Agaropectin, the non gelling fraction, is a sulfated polysaccharide (3% to 10% sulfate), composed of agarose and varying percentages of ester sulfate, D-glucuronic acid, and small amounts of pyruvic acid. The proportion of these two polymers varies according to the species of seaweed. Agarose normally represents at least two-thirds of the natural agar-agar.
Gelling Properties of Agar
The gelling portion of agar-agar has a double helical structure. Double helices aggregate to form a three-dimensional structure framework which holds the water molecules within the interstices of the framework. Thus, thermo-reversible gels are formed. The gelling property of agar-agar is due to the three equatorial hydrogen atoms on the 3,6-anhydro-L-galactose residues, which constrain the molecule to form a helix. The interaction of the helixes causes the formation of the gel.
Regarding its gelling power, agar-agar is outstanding among other hydrocolloids. Agar-agar gels can be formed in very dilute solutions, containing a fraction of 0.5% to 1.0% of agar-agar. These gels are rigid, brittle, have well defined shapes, as well as sharp melting and gelling points. Moreover, they clearly demonstrate the interesting phenomenon of syneresis (spontaneous extrusion of water through the surface of the gel), and hysteresis (temperature interval between melting and gelling temperatures). Gelling occurs at temperatures far below the gel melting temperature. A 1.5% solution of agar-agar forms a gel on cooling to about 32º to 45º C that does not melt below 85º C. This hysteresis interval is a novel property of agar-agar that finds many uses in food applications. The gel strength of the agar-agar is influenced by concentration, time, pH, and sugar content. The pH noticeably affects the strength of the agar gel; as the pH decreases, the gel strength weakens. Sugar content has also a considerable effect over agar gel. Increasing levels of sugar make gels with harder but less cohesive texture.
Agar Hydration
In order for agar to work successfully it first needs to hydrate, or absorb water. To properly hydrate agar it must be brought to a boil at 212°F (100°C) and simmered for 3 to 5 minutes.
Agar does not hydrate well in acidic liquids, making gelling difficult. To get around this issue, first hydrate the agar in a neutral liquid and then add it to the acidic liquid. Agar Will form gels at 88 F and does not melt below 136 F. Agar is a gel at room temperature, remaining firm at temperature as high as 65°C. Agar melts at approximately 85°C, a different temperature from that at which it solidifies, 32-40°C. This property is known as hysteresis.
Agar exhibits hysteresis, melting at 85 °C (358 K, 185 °F) and solidifying from 32–40 °C (305–313 K, 90–104 °F). This property lends a suitable balance between easy melting and good gel stability at relatively high temperatures.
Uses in Cooking
Agar is used as a thickening agent for soups, fruits preserves, ice cream, sauces, jelly-based desserts, custards, puddings and other tasty treats. Agar easily gels most liquids and the gels can range from soft to hard, depending on the amount used.Agar can also be used to make dense foams when used in an ISI foamer.
Readings and References
Chemical Structure and Physio-Chemical Properties of Agar
PolySac3DB: an annotated data base of 3 dimensional structures of polysaccharides

