Chemical compounds in coffee that produce aroma and bitterness
Coffee is know to contain over 1000 chemical compounds that contribute to both the taste and aroma of coffee. Although Caffeine is the most well know molecule and although is a bitter compound, it only contributes about 15% of coffee's bitter compounds. Caffeine does however bind to adenosine receptors in the brain which is causes a stimulant effect.
Phenolic Compounds found in Coffee
Phenolic compounds are ubiquitous constituents of higher plants found in a wide range of commonly consumed plant foods including coffee. These compounds are secondary metabolites of plants generally involved in defense against ultraviolet radiation, and as natural animal toxicants and pesticides against invading organisms.
While condensed tannins are the main phenolic compounds in coffee pulp, in the seed, phenolic compounds are present predominantly as a family of esters formed between certain hydroxycinnamic acids and quinic acid, collectively known as chlorogenic acids (CGA). CGA, which are present in high concentrations in green coffee seeds (up to 14 %), have a marked influence in determining coffee quality and play an important role in the formation of coffee flavor. See the abstract below and full review article for more details on this topic.
From: Phenolic compounds in coffee - Brazilian Journal of Plant Physiology - Adriana Farah; Carmen Marino Donangelo
Abstract: "Phenolic compounds are secondary metabolites generally involved in plant adaptation to environmental stress conditions. Chlorogenic acids (CGA) and related compounds are the main components of the phenolic fraction of green coffee beans, reaching levels up to 14 % (dry matter basis). These compounds have a number of beneficial health properties related to their potent antioxidant activity as well as hepatoprotective, hypoglycemic and antiviral activities. The main groups of CGA found in green coffee beans include caffeoylquinic acids, dicaffeoylquinic acids, feruloylquinic acids, p-coumaroylquinic acids and mixed diesters of caffeic and ferulic acids with quinic acid, each group with at least three isomers. During coffee processing, CGA may be isomerized, hydrolyzed or degraded into low molecular weight compounds. The high temperatures of roasting also produce transformation of part of CGA into quinolactones and, along with other compounds, melanoidins. This review focuses on the chemical characteristics, biosynthesis, and distribution of CGA and related compounds in coffee. The influence of genetic, physiological and environmental factors as well as processing on the chemical composition of coffee beans is discussed. The impact of CGA composition of green coffee on cup quality is also approached. Despite the existence of substantial published information on the total levels of CGA in coffee, more research is needed on the composition of minor phenolic compounds and specific CGA isomers (and related substances) in green and roasted coffee beans, as well as their impact on coffee quality." Full Review article available online
What causes the bitterness found in coffee?
In a research study done by Thomas Hofmann, Ph.D., a professor of food chemistry and molecular sensory science at the Technical University of Munich in Germany.-- the main source of bitterness is from two sources: chlorogenic acid lactons and pheylindanes. Both the lactones as well as the phenylindanes are derived from chlorogenic acid, which is not itself bitter. Chlorogenic acid is found in the green unroasted coffee bean.
According to Hoffman, roasting is the key factor driving the bitter taste in coffee beans. So the stronger you roast the coffee, the more harsh itwill tends to get. We've known for some time that the chlorogenic acid lactones are present in coffee, but their role as a source of bitterness was not known until now," Hofmann says. Ironically, the lactones as well as the phenylindanes are derived from chlorogenic acid, which is not itself bitter.
Screening and identification of bitter compounds in roasted coffee brew by taste dilution analysis -Developments in Food Science- Oliver Franka, Gerhard Zehentbauerb, Thomas Hofmann (2007)
Abstract: Intense bitter taste compounds were identified in roasted coffee brew using sensory-guided fractionation, LC-MS/MS and 1D/2D-NMR spectroscopy, syntheses, and model roasting experiments with potential precursors. The intense bitter tastants of coffee were 3-O-caffeoyl-γ-quinide (1), 4-O-caffeoyl-γ-quinide (2), 4-O-caffeoyl-muco-γ-quinide (3), 5-O-caffeoyl-muco-γ-quinide (4), 5-O-caffeoyl-epi-δ-quinide (5), 3-O-feruloyl-γ-quinide (6), 4-O-feruloyl-γ-quinide (7) 3,4-O-dicaffeoyl-γ-quinide (8), 4,5-O-dicaffeoyl-muco-γ-quinide (9) and 3,5-O-dicaffeoyl-epi-δ-quinide (10). Determination of the bitter taste recognition thresholds showed that, depending on their chemical structure, the bitter threshold concentrations ranged between 9.8 and 180 μmol/l (water). Quantification and determination of the dose over threshold factors for the individual bitter compounds revealed that approximately 80% of the bitterness of the decaffeinated coffee beverage could be related to these 10 quinides.
Phenylindanes, which are the chemical breakdown products of chlorogenic acid lactones, are found at higher levels in dark roasted coffee, including espresso. These chemicals exhibit a more lingering, harsh taste than their precursors, which helps explain why dark-roasted coffees are generally more bitter, Hofmann says.
Using advanced chromatography techniques and a human sensory panel trained to detect coffee bitterness, Hofmann and his associates found that coffee bitterness is due to two main classes of compounds: chlorogenic acid lactones and phenylindanes, both of which are antioxidants found in roasted coffee beans. The compounds are not present in green (raw) beans, the researchers note.
The lactones derive from chlorogenic acids, also referred to as O-caffeoylquinic acids, which are the predominant polyphenols present in raw coffee beans. Bean roasting either can break these phenolic acids down to form di- and trihydroxybenzenes such as hydroxyhydroquinone or can epimerize and dehydrate the acids to give various lactones that provide a "pleasant, coffeelike bitter taste quality" in light- to medium-roast coffee, Hofmann said.
Thomas Hofmann and his research associate Oliver Frank of Germany's University of Münster turned to fractionation experiments to try to isolate the bitter factors. They found that two groups of components were significant contributors to bitterness: chlorogenic acid lactones and multiply hydroxylated phenylindanes. The lactones derive from chlorogenic acids, also referred to as O-caffeoylquinic acids, which are the predominant polyphenols present in raw coffee beans. Bean roasting either can break these phenolic acids down to form di- and trihydroxybenzenes such as hydroxyhydroquinone or can epimerize and dehydrate the acids to give various lactones that provide a "pleasant, coffeelike bitter taste quality" in light- to medium-roast coffee, Hofmann said.
What is chlorogenic acid? Chlorogenic acid is a natural chemical compound which is the ester of the phenolic acids caffeic acid and (−)-quinic acid.
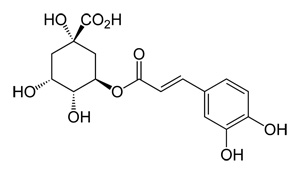
Chlorogenic Acid Structure
Of all plant constituents, coffee has one of the highest concentrations of chlorogenic acids. Whenroasting coffee, some of these are transformed into chlorogenic acid lactones (CGL). Note: the term chlorogenic acids can also refer to a related family of esters of hydroxycinnamic acids (caffeic acid, ferulic acid and p-coumaric acid) with quinic acid. Green coffee beans contain the largest amounts of CGA foundin plants, ranging from 6 to 12%
Chlorogenic acid lactones, which include about 10 different chemicals in coffee, are the dominant source of bitterness in light to medium roast brews. 5 - caffeoylquinic acid is the most prevalemt of the chlorgenic acids in green coffee beans
According to Adriana Farah, Vanderbilt Institute for Coffee Studies, Vanderbilt University School of Medicine--"Of all plant constituents, coffee has one of the highest concentrations of chlorogenic acids. When roasting coffee, some of these are transformed into chlorogenic acid lactones (CGL). We have studied the formation of CGL during the roasting of coffee beans..." see:
Effect of Roasting on the Formation of Chlorogenic Acid Lactones in Coffee
Seven Chlorogenic Acid Lactones were identified: 3-caffeoylquinic-1,5-lactone (3-CQL), 4- caffeoylquinic-1,5-lactone (4-CQL), 3-coumaroylquinic-1,5-lactone (3-pCoQL), 4-coumaroylquinic-1,5-lactone (4-pCoQL), 3-feruloylquinic-1,5-lactone (3-FQL), 4-feruloylquinic-1,5-lactone (4-FQL), and 3,4-dicaffeoylquinic-1,5-lactone (3,4-diCQL). 3-CQL was the most abundant lactone in C. arabica and C. canephora, reaching peak values of 230 ± 9 and 254 ± 4 mg/100 g (dry weight),
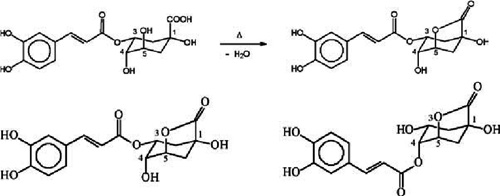
Formation of a 1,5- γ -quinolactone from chlorogenic acid during roasting (A), and the two major chlorogenic acid lactones in roasted coffee: 3-caffeoylquinic-1,5- γ -lactone (left) and 4-caffeoylquinic-1,5- γ -lactone (right) (B) Although under IUPAC rules the numbering system for the lactones is different from that for the acids, in order to avoid confusion, most reports in the literature (including the present work) have been using for lactones the same numbering of the carbons as for the acid precursors. Image from: Phenolic compounds in coffee - Brazilian Journal of Plant Physiology - Adriana Farah; Carmen Marino Donangelo.
Green coffee beans actually contain up to 7 percent of chlorogenic acid. Regular coffee beans lose some of the amount they contain after the beans have been roasted.
During roasting, CGA's slowly decompose to form caffeic and quinic acid with about 50% of the original CGA being destroyed in a medium roast.
What gives coffee its aroma?
According to Thomas Hoffman there are a thousand volatile compounds that have been identified in coffee, though just 40 or so of these substances "have been demonstrated to contribute to the alluring smell". They include β-damascenone (which has an aroma like cooked apples), 2-furfurylthiol (sulfury, roasty), 2-isobutyl-3-methoxypyrazine (earthy), guaiacol (spicy), 2,3-butanedione (buttery), and 4-hydroxy-2,5-dimethyl-3(2H)-furanone (caramel-like). The flavor and aroma compounds derive from multiple chemical reactions, including the Maillard reaction, caramelization, polyphenol degradation, polymerization of carbohydrates, and pyrolysis. see: Tweaking Coffee's Flavor Chemistry --Chemical and Engineering News.
Phenylindanes, which are the chemical breakdown products of chlorogenic acid lactones, are found at higher levels in dark roasted coffee, including espresso. These chemicals exhibit a more lingering, harsh taste than their precursors, which helps explain why dark-roasted coffees are generally more bitter according to Thomas Hofmann.
The type of brewing method used can also influence the perception of bitterness. Espresso-type coffee, which is made using high pressure combined with high temperatures, tends to produce the highest levels of bitter compounds. While home-brewed coffee and standard coffee shop brews are relatively similar in their preparation methods, their perceived bitterness can vary considerably depending on the roasting degree of the beans, the amount of coffee used, and the variety of beans used.
About Coffee Yields
Although some reports are as high as 28%--(Roasted coffee beans are ~28% (by weight) water-soluble. This means that you can extract ~28% of the coffee bean's mass in water. The rest is pretty much cellulose and plant stuff that forms the structure of the seed.
Yields of under 18% are "under-extracted", specifically "under-developed" – desirable components have not been sufficiently extracted – and "unbalanced", specifically sour, because acids are extracted early, while balancing sugars (sweetness) and bitter components are extracted later.
Yields of over 22% are "over-extracted", specifically bitter, as bitter components continue to be extracted after acids and sugars have largely completed extraction. In certain situations, yields surpassing 22% can be absent of bitterness.
Caffeine is extracted early, so higher yields do not yield more caffeinated coffee, only over-extracted coffee.
READINGS AND REFERENCES
Coffee Roasting Chemistry: Chlorogenic Acids - SCAA
Tweeking Coffees Flavor Chemistry - American Chemical Society
Coffee Chemistry - Chlorogenic Acid
Coffee Chemistry: Coffee Aroma
Phenolic compounds in coffee - Brazilian Journal of Plant Physiology - Adriana Farah; Carmen Marino Donangelo
The Chemistry of Coffee - Why is Coffee Bitter - Compoundchem.com
Battling bitter coffee -- chemists vs. main source of coffee bitterness - AAAS
Effect of Roasting on the Formation of Chlorogenic Acid Lactones in Coffee
Screening and identification of bitter compounds in roasted coffee brew by taste dilution analysis - Thomas Hoffman
Related Coffee Sites
See also:
Chemical compounds in coffee that produce aroma and bitterness
Science behind pulling the perfect espresso shot
Health Risks in Cooking
What is the difference between LDL and HDL?
What are the different types of Omega-3 fatty acids?
What is the difference between nitrates and nitrites?
What is the difference between saturated and unsaturated fats?
Science of Chocolate

NEWScience of ChocolateHow is Chocolate Made?
What are the health benefits of Chocolate?
What are the drugs in Chocolate?