Chemical and Physical Properties of Pectin in Cooking
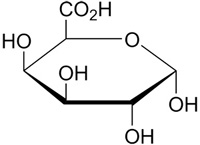
PectinPectin is a structural polysaccharide that is integral for the stability of plant cell walls. The biological function of pectin is to cross-link cellulose and hemicellulose fibers, providing rigidity to the cell wall. Pectin is also a major component of the middle lamella, where it helps to bind cells together. Molecular Structure of Pectin Galacturonic acid is a sugar acid, an oxidized form of D-galactose (a C-4 epimer of glucose). It is the main component of pectin, in which it exists as the polymer polygalacturonic acid
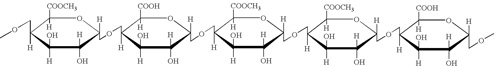
Pectin contains a polysaccharide backbone with an a-(1 -->4)-linked D-galacturonic acid. The acid groups along the chain are largely esterifed with methoxy groups in the natural product. There can also be acetyl groups present on the free hydroxy groups.
Gelling Properties of Pectin A pectin-gel can be formed when the ingredients are heated, thereby dissolving the pectin. Upon cooling below gelling temperature, a gel starts to form. If gel formation is too strong, syneresis --the extraction of the liquid component-- occurs possibly producing a granular texture. Weak gelling leads to excessively soft gels. Pectins gel according to specific parameters, such as sugar, pH and bivalent salts (especially Ca2+). In high-ester pectins at soluble solids content above 60% and a pH-value between 2.8 and 3.6, hydrogen bonds and hydrophobic interactions bind the individual pectin chains together. These bonds form as water is bound by sugar and forces pectin strands to stick together. These form a 3-dimensional molecular net that creates a gel. In low-ester pectins, ionic bridges are formed between calcium ions and the ionised carboxyl groups of the galacturonic acid. Low-ester pectins need calcium to form a gel, but can do so at lower soluble solids and higher pH-values than high-ester pectins. In the kitchen HM (high methoxyl) pectin produces a theroirreversible gel that will not melt when subject to high temperature. The optimal concentrations for gel formation are:
Pectin Hydration The functional properties of pectins in food systems arise because of their ability to form crosslinked polymer networks that are highly hydrated. How is Pectin used in Cooking The main use for pectin (vegetable agglutinate) is as a gelling agent, thickening agent and stabilizer in food. The classical application is giving the jelly-like consistency to jams or marmalades, which would otherwise be sweet juices. Pectin also reduces syneresis in jams and marmalades and increases the gel strength of low calorie jams. For household use, pectin is an ingredient in gelling sugar (also known as "jam sugar") where it is diluted to the right concentration with sugar and some citric acid to adjust pH. You find dry-regular pectin in just about any processed food that contains a lot of sugar because it binds with glucose to thicken. No-sugar pectin binds to calcium, but only 10 to 30 milligrams -- less than a tablespoon of milk -- making it the best choice when thickening savory sauces. Pectin is a good alternative to starch-based thickeners, too. It doesn't alter the taste and only needs about one minute to work. In some countries, pectin is also available as a solution or an extract, or as a blended powder, for home jam making. For conventional jams and marmalades that contain above 60% sugar and soluble fruit solids, high-ester pectins are used. With low-ester pectins and amidated pectins less sugar is needed, so that diet products can be made. Readings and References Chemistry and uses of pectin-- a review Water Structure and Science -- Pectin An Introduction to Pectin - Structure Pectin Basics - Gelling properties and applications |
|
Science of Cooking
See also: